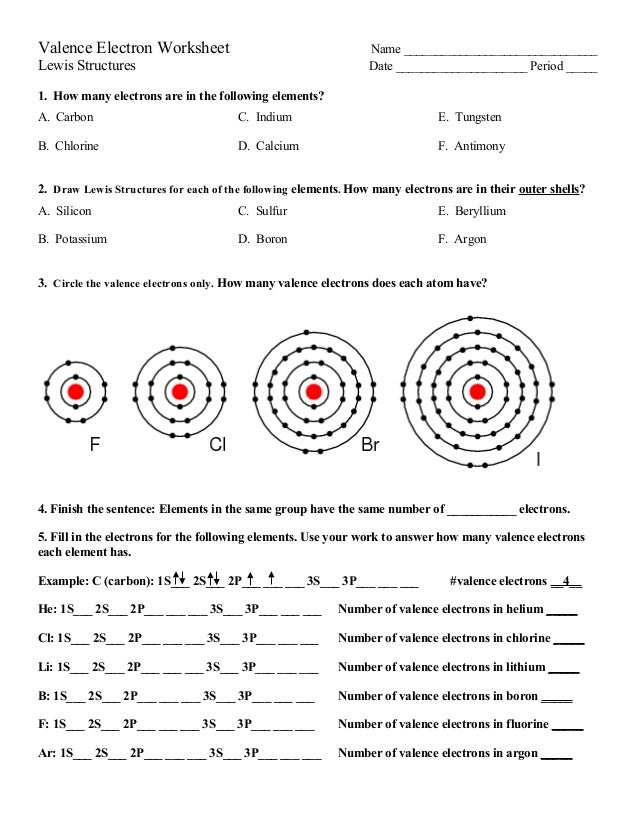
However, the number of neutrons in the nuclei of the calcium atoms can vary. Atoms of the same element with different numbers of neutrons are isotopes. There are many isotopes of calcium, ranging from calcium-40 with 20 neutrons, to calcium-48 with 28 neutrons. Rar software for mac os x. For more on the isotopes of calcium, refer to the following website. Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n2) electrons.
Click to see full answer
 Then, why is a calcium ion smaller than a calcium atom?
Then, why is a calcium ion smaller than a calcium atom?Because the calcium ion donates two electrons to achieve the stable state. Thus, the radius of a calcium ion is smaller than a calcium atom.
Secondly, how does a calcium atom become an ion? When it loses electron(s) it becomes positively charged and is called a cation. Calcium atom with electron arrangement K (2),L(8),M(8),N(2) loses two electrons from its outermost shell ( N shell) and forms positive ions called Calcium , Ca2+ ion.
Winzip keygen for mac. Then, what does calcium ion do?
Calcium in biology. Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms cell. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization.
What does a calcium atom look like?
Calcium atoms have 20 electrons and 20 protons. There are 2 valence electrons in the outer shell. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust. Under standard conditions calcium is a shiny, silvery metal.
| General | |
|---|---|
| Symbol | 48Ca |
| Names | calcium-48, Ca-48 |
| Protons | 20 |
| Neutrons | 28 |
| Nuclide data | |
| Natural abundance | 0.187% |
| Half-life | (6.4+0.7 −0.6+1.2 −0.9) × 1019 a |
| Isotope mass | 47.952534(4) u |
| Isotopes of calcium Complete table of nuclides | |
Calcium-48 is a scarce isotope of calcium containing 20 protons and 28 neutrons. It makes up 0.187% of natural calcium by mole fraction.[1] Although it is unusually neutron-rich for such a light nucleus, its beta decay is extremely hindered, and so the only radioactive decay pathway that it has been observed to undergo is the extremely rare process of double beta decay. Its half-life is about 6.4×1019 years,[2] so for all practical purposes it can be treated as stable. One factor contributing to this unusual stability is that 20 and 28 are both magic numbers, making 48Ca a 'doubly magic' nucleus.
Number Of Electrons In Calcium Atom
Since 48Ca is both practically stable and neutron-rich, it is a valuable starting material for the production of new nuclei in particle accelerators, both by fragmentation[3] and by fusion reactions with other nuclei, for example in the discoveries of the heaviest five elements on the periodic table, from flerovium to oganesson.[4] Heavier nuclei generally require a greater fraction of neutrons for maximum stability, so neutron-rich starting materials are necessary.
48Ca is the lightest nucleus known to undergo double beta decay and the only one simple enough to be analyzed with the sdnuclear shell model. It also releases more energy (4.27 MeV) than any other double beta decay candidate.[5] These properties make it an interesting probe of nuclear structure models and a promising candidate in the ongoing search for neutrinoless double beta decay.
See also[edit]
References[edit]
Number Of Electrons In Calcium-40
- ^Coursey, J. S.; D. J. Schwab; R. A. Dragoset (February 2005). 'Atomic Weights and Isotopic Compositions'. NIST Physical Reference Data. Retrieved 2006-10-27.CS1 maint: discouraged parameter (link)
- ^Arnold, R.; et al. (NEMO-3 Collaboration) (2016). 'Measurement of the double-beta decay half-life and search for the neutrinoless double-beta decay of 48Ca with the NEMO-3 detector'. Physical Review D. 93 (11): 112008. arXiv:1604.01710. Bibcode:2016PhRvD.93k2008A. doi:10.1103/PhysRevD.93.112008.
- ^Notani, M.; et al. (2002). 'New neutron-rich isotopes, 34Ne, 37Na and 43Si, produced by fragmentation of a 64A MeV 48Ca beam'. Physics Letters B. 542 (1–2): 49–54. Bibcode:2002PhLB.542..49N. doi:10.1016/S0370-2693(02)02337-7.
- ^Oganessian, Yu. Ts.; et al. (October 2006). 'Synthesis of the isotopes of elements 118 and 116 in the 249Cf and 245Cm + 48Ca fusion reactions'. Physical Review C. 74 (4): 044602. Bibcode:2006PhRvC.74d4602O. doi:10.1103/PhysRevC.74.044602.
- ^Balysh, A.; et al. (1996). 'Double Beta Decay of 48Ca'. Physical Review Letters. 77 (26): 5186–5189. arXiv:nucl-ex/9608001. Bibcode:1996PhRvL.77.5186B. doi:10.1103/PhysRevLett.77.5186. PMID10062737.
